
October 12, 2022, by Mei Kee Lee
Student Sharing: My Industrial Internship Experience at Sunward Pharmaceutical – Chong Hui Ting & Chong Min Jie
Article written by Chong Hui Ting and Chong Min Jie, Pharmaceutical and Health Sciences Year 3 students. They worked as an intern at Sunward Pharmaceutical Sdn. Bhd during summer break.
___________________________________________________________________________
Sunward Pharmaceutical is one of the largest generic pharmaceutical manufacturers in Singapore with factories in Malaysia and Thailand. Sunward Pharmaceutical manufactures pharmaceutical products of dosage forms such as liquids for ingestion, creams, plain and coated tablets.

Chong Hui Ting:
How did I find the intern position?
As we know, Dr Lee Mei Kee used to share the lists of internship opportunities offered by UNM School of Pharmacy and external parties with us through email. When I received the email, I started thinking what to do with my summer break. I decided to apply for an internship position in pharmaceutical or cosmetic industries. Furthermore, my friend, Min Jie also got the lists of pharmaceutical industries from her tutor, Dr Tahir. I also seek for the help from UNM Career Advisory Service (CAS). Mr Kamil from CAS had provided me some suggestions and company recommendations based on my interest. Without further ado, I started to search for an internship position in these pharmaceutical or cosmetic industries and emailed my resume to them. Fortunately, I received a call from Sunward Pharmaceutical’s Human Resource Department few days after sending my application. After a brief conversation, she sent me an email with a job application form. Finally, I received my Letter of Offer from Sunward Pharmaceutical at the end of April.
What did I learn?
Working as a Production Intern at Sunward Pharmaceutical had been a great experience for me. Throughout the internship, I had improved my skills in using different instruments including tablet thickness tester, hardness tester, moisture analyzer and many others to ensure the quality of the in-process products. Besides, I got to enhance my problem-solving skills in rectifying the issues occurred during the manufacturing process since challenges and problems are inevitable in large scale manufacturing. For example, dry granules might lead to tablet capping and chipping whereas wet granules tend to stick punches during tableting process. I required to report these situations to the supervisors and discussed the immediate actions with them to rectify the problems. There’s no denying that my practical knowledge was put to good use here because I was able to apply the knowledge that I learnt during my first and second year of study. For instance, Pharmaceutics 1: Physicochemical Science and Medicines Design and Pharmaceutics 2: Pharmaceutical Technology are the primary modules which help me to understand the process in formulating the pharmaceutical products. Different parameters of the machine such as speed of the mixer, temperature and drying time will also affect the quality of the finished products. Furthermore, I had learnt the formulations and special precautions for different products by reading through Batch Manufacturing Record (BMR). It indeed broadened my horizons about different drug formulations. Moreover, I was lucky to meet the production pharmacists and operators here as they were very friendly and willing to answer my questions. Moreover, operator also taught us how to operate machines such as High Shear Mixer, fluid beg dryer, tableting machine and many others.
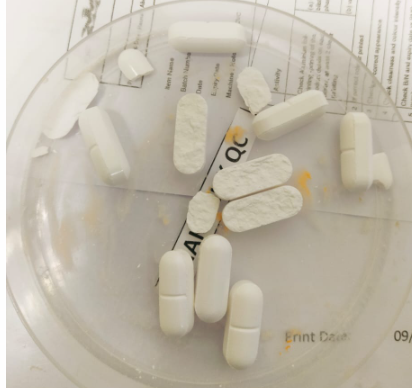
Tablet capping with friability more than 1%.
Chong Min Jie:
What is my internship job scope?
Hui Ting and I were assigned to work under the Production Department in Sunward Pharmaceutical. As a production intern, we got the opportunity to observe and monitor how tablets, syrups and creams being manufactured in a large scale for commercial use. In the production area of oral solid dosage forms, we observed the processes such as granulation, drying, blending, tableting, film-coating, sugar coating and blister packing of tablets. In galenical area, we monitored the processes such as syrup preparation, syrup filling and capping as well as cream preparation. When monitoring the processes, we were required to record the parameters and carried out some in-process quality checks (IPQC) including measurement of tablet weight, thickness, hardness and moisture content. The purpose of IPQC is to ensure the quality of the finished products. Sometimes, we were in-charge of recording the amount of finished products inside the bulk intermediate room and making a report to production pharmacist. This can ease their jobs in arranging the date of export. Moreover, we were able to communicate with colleagues from Quality Control (QC), Quality Assurance (QA), maintenance and store departments to get the information from them in furnishing our daily tasks. Hence, good teamwork and communication skills are very important to work harmonically and effectively with colleagues. Occasionally, we will stay in the office to complete the daily backlog report (Report that recorded the daily activities in the production site).

🌟Shiny tablets after polishing (Final step of sugar coating process)
Tips for the successful internship
There’s no doubt that resume is a must when applying for internship. Hence, remember to update your resume from time to time! You can also register an LinkedIn account as LinkedIn is one of the most useful platform for searching a job. Engage in networking can help you to explore different career opportunities, so don’t miss out.
I still remember that kind of nervousness and excitement that I experienced on my first day of internship. I believe most of us will have the same feeling as it’s perfectly normal to be nervous around new situations and new people. Hence, as a piece of advice for my juniors who might be anxious before the start of internship, I hope that you will study the background of your company beforehand as this allows you to be more prepared. Before heading to the workplace for an internship, you can always seek for help from your senior who is currently employed there through LinkedIn or any other platforms if there’s any. For me, I asked Ms Nabihah who is my senior in PHS course yet the Quality Assurance Officer in Sunward Pharmaceutical about the company and things that I need to take note of. As an intern in production department, you will definitely face many unexpected situations during the manufacturing process. Hence, you must be calm and don’t nervous in rectifying the problems. Finally, don’t stay silent and unsure if you’re in doubt, be brave to ask questions as this is the only way to solve your problems!
-
Post a comment
